- funding Stage : Series A
- Raising Amount : KRW 3B
- Desired Fundraising Timeframe : 3Q 2024
As smartphone usage continues to grow, so does the number of people suffering from eye conditions. One of the most common issues is dry eye syndrome, which can be a warning sign for more serious eye health problems. With eyes constantly exposed to various stimuli, it’s clear that the prevalence of eye diseases will only increase.
At the center of the eye is the retina, where yellow macular cells are located. As multiple factors, such as aging and eye diseases, progress, these macular cells can become damaged, leading to Age-related Macular Degeneration (AMD). As eye diseases worsen or as aging advances, waste deposits called drusen accumulate in the retinal epithelial cells. If these deposits aren’t cleared in time, new blood vessels can form in the retina, potentially causing blindness.
Eye conditions like dry eye syndrome and AMD are difficult to treat fundamentally, and even with treatment, many patients do not see significant improvements. Treating AMD, in particular, often involves injecting medication directly into the eye, a process that is both frightening and uncomfortable for patients.
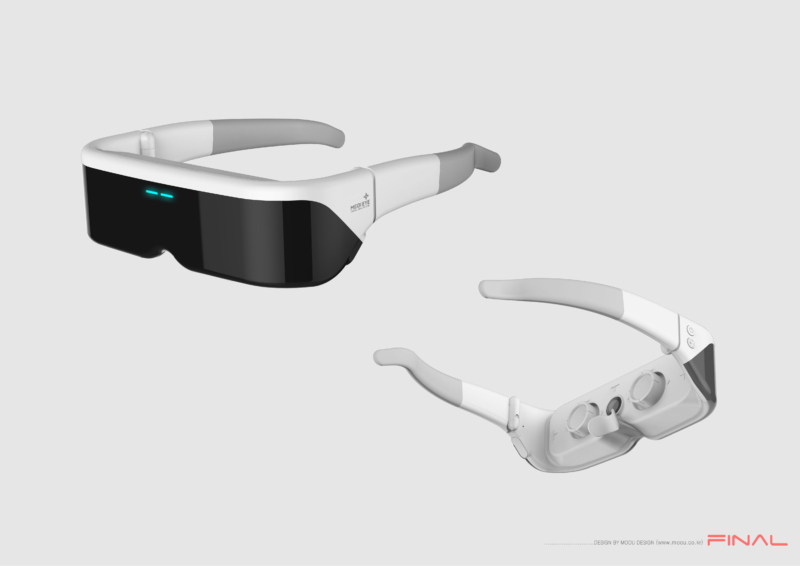
However, there is a company that aims to address these eye health challenges using light. Medi-IoT, based at the Seoul Bio Hub Global Center, is developing treatments for eye diseases through a technology called Photo-Bio-Modulation (PBM). Using this technology, they have created an electronic therapeutic device called “MEDI-EYE,” designed in the form of sunglasses for greater comfort and ease of use.

MEDI-EYE emits a combination of three specific light sources from four LEDs, tailored to the type of eye disease and the patient’s condition, which are then directed at the eyes. This light stimulates the mitochondria in the cells, boosting the production of ATP and reactive oxygen species, which activates cellular functions. This process helps treat eye conditions and prevents further deterioration. Medi-IoT is currently preparing to begin clinical trials for MEDI-EYE aimed at treating dry eye syndrome and macular degeneration. If all goes well, they plan to enter the medical market by 2026, with wellness products designed to promote eye health launching as early as August this year.
So, how effective is MEDI-EYE in treating eye conditions? Medi-IoT reports that in preliminary trials, a patient who had lost vision due to retinitis pigmentosa regained enough sight to recognize bus numbers after using MEDI-EYE. Buoyed by this success, the company is now seeking Series A investments.
Interestingly, the leadership at Medi-IoT doesn’t come from a medical background but rather from engineering. CEO Sang-Geun Lee holds a PhD in engineering from Georgia Tech and has experience developing imaging and signal processing technologies at Samsung Research America. Co-CEO Seung-Bin Baek is a seasoned entrepreneur with a history of successful exits in the tech industry.
That said, Medi-IoT is not without medical expertise. CEO Lee describes Medi-IoT as “an advanced startup researching eye disease treatments based on photonic convergence technology, supported by a group of key doctors, specialized researchers, and shareholder physicians who provide technical expertise.” The company is confident that combining engineering and medicine will generate powerful synergies.
With the dramatic increase in smartphone usage, the constant stream of visual information, advancements in virtual and augmented reality (XR), and the growing elderly population, the demand for eye disease treatments is only going to rise. Medi-IoT, ahead of the curve, is poised for an exciting journey into the future of eye care.

What problems is Medi-IoT trying to solve?
Medi-IoT is addressing the lack of effective treatments for dry eye syndrome and macular degeneration. Our product, MEDI-EYE, is an electronic therapeutic device that uses Photo-Bio-Modulation (PBM) technology to treat these conditions. It combines four light sources to deliver a non-invasive, side-effect-free treatment. MEDI-EYE is designed as ultra-lightweight smart glasses, tailored for user comfort through detailed ergonomic analysis.
The device features an advanced light control system that combines different light sources based on time and intensity settings, ensuring uniform exposure. Our real-time monitoring system, equipped with cutting-edge computer vision technology, ensures the uniformity of the light even for invisible wavelengths.
Currently, dry eye syndrome lacks a fundamental cure, and treatments often lead to issues such as resistance, burning sensations, itching, and decreased vision. As a result, these treatments are typically used only for temporary symptom relief, similar to painkillers, leading 25-50% of patients to discontinue treatment.

Macular degeneration, caused by damage to the central macular cells in the retina, often worsens with age. Symptoms include eye pain, retinal bleeding, increased intraocular pressure, and inflammation. The standard treatment involves anti-VEGF injections into the eye to slow disease progression, but these injections are inconvenient, painful, and require regular administration. This leads to higher treatment costs and potential side effects, limiting effectiveness. Around 35-50% of patients do not achieve the desired results with current anti-VEGF therapies. Medi-IoT aims to address these issues with MEDI-EYE.
How does Medi-IoT solve this problem?
MEDI-EYE uses PBM (Photo-Bio-Modulation) technology to deliver light energy to the eyes. This energy targets the mitochondria in cells, particularly retinal cells, and receptors in cell membranes, boosting the production of reactive oxygen species (ROS) and adenosine triphosphate (ATP). This process enhances cellular activity, healing retinal pigment epithelial (RPE) cells, and removes impurities from the retina, thereby treating conditions like dry AMD and macular edema.
As people age or experience inflammation, drusen—a substance that accelerates AMD—can accumulate in the retina. Medi-IoT’s multi-wavelength technology activates cells through mechanisms in photoreceptors, RPE (Blood-Retina Barrier BRB-2), and Bruch’s membrane (BRB-1), effectively removing drusen.
Recent advancements in optical technology, including fiber lasers, laser diodes, LEDs, and OLEDs, have led to evolving treatment devices. PBMT (Photo-Bio-Modulation Therapy) has demonstrated its effectiveness, and Medi-IoT combines these optical and biotechnology advancements to treat eye diseases.
What are Medi-IoT’s competitive and technological advantages?
Medi-IoT developed MEDI-EYE using advanced light sources and optical modules with a focus on ultra-lightweight design. Our On-Device AI model ensures optimal optical focusing, and our compact optical lenses are designed for efficient LED arrangements.
Our optical module, capable of combining multi-wavelength light sources, provides superior treatment compared to traditional single-wavelength methods. The product is designed to ensure light reaches the retina without loss. By using multi-wavelength LEDs, we offer flexible wavelength combinations based on treatment needs, while minimizing heat to reduce risks to users.
What products and services does Medi-IoT offer, and what is their current status?
We have developed MEDI-EYE, a next-generation, innovative medical device designed to transform eye disease treatment. This ultra-lightweight smart glasses-type device offers convenient and effective treatment. With ongoing technological improvements, we’ve reduced treatment times and maximized efficiency. The device operates for over two hours on a single charge, is lightweight, and easy to use.
MEDI-EYE is expected to receive KFDA approval and launch in 2026. The wellness version of the product was prototyped in June and will be released once KC, FCC, SRCC, and CE certifications are completed in August.

What is the size of Medi-IoT’s target market, and who are its key target customers?
The global market for ophthalmic pharmaceuticals related to dry eye syndrome was valued at $4.8 billion in 2019 and is projected to grow at a compound annual growth rate (CAGR) of 4.7%, reaching $6.9 billion by 2027. The global market for eye drops was valued at $3.9 billion in 2018 and is expected to grow at a CAGR of 6.14%, reaching $5.6 billion by 2026. In South Korea, the market was estimated at 200 billion KRW in 2018 and 300 billion KRW in 2020. Although 80% of people experience dry eye syndrome, awareness of symptoms, treatment options, and related complications remains low.
According to Verified Market Research, the global electronic drug market was valued at $24 billion in 2020 and is expected to grow at a CAGR of 8.9%, reaching $47 billion by 2026.
Medi-IoT plans to start promoting in international markets by 2025 and anticipates revenue generation by 2026, when the market size is expected to be even larger. IDTechEx predicts the electronic drug market will grow by over 10% annually, and the retinal treatment market will also expand rapidly.

What is Medi-IoT’s business model?
Our business model starts with early entry into the wellness market for eye health management, followed by expansion into electronic therapeutic devices for eye disease treatment. We aim to maximize profits through diversified sales channels, including product sales and subscription models.
For wellness products, we plan to participate in procurement programs for public enterprises and institutions, with rapid entry expected through startup procurement systems. We will also target the initial market through employee assistance programs (EAP) in corporate welfare malls and distribution channels like Olive Young, focusing on students, office workers, and professionals.
For medical devices, we plan to secure manufacturing licenses and insurance coverage to enter hospitals and expand into specialized markets for personalized treatment.
What achievements has the Medi-IoT team made so far?
Medi-IoT holds 9 patents related to eye disease treatments (2 registered, 2 PCT, 5 pending) and has published two SCI-level journal papers (JQR Q1, IF 5.6) on non-clinical trial results (for dry eye syndrome and dry AMD).
Our PBM non-clinical trial data was presented at the Society for Neuroscience 2023, one of the world’s largest scientific conferences. Medi-IoT also won first place at the 2022 KU Tech Fair, receiving full sponsorship for CES 2023. We have received recognition at the 2024 Hong Kong InnoEX Innovation Competition (40 million KRW in prize money) and participated in various international exhibitions, including the Radical Healthcare Festival in Finland.
We have completed KC certification for wellness products (general goods), and GMP inspections and certification for exploratory/confirmatory clinical trials are ongoing (including electromagnetic tests, electrical/mechanical safety, usability, performance, and photobiological safety).

What is the competitive strength of the Medi-IoT team?
Our team comprises experts in various development fields, H/W development, and technology commercialization. We have brought in specialists to enhance product development and business expansion.
CEO Sang-Geun Lee, an expert in imaging, signal processing, and electronic drugs, has a PhD from Georgia Tech and previously worked at Samsung Research America. Co-CEO Seung-Bin Baek, with over 20 billion KRW in exit experience from three ventures, is a leader in technology commercialization. Our advisory board includes key doctors and shareholders specializing in eye disease treatments based on photonic convergence technology, along with specialized researchers and shareholder physicians.
How has the Seoul BioHub program helped Medi-IoT’s growth?
Joining the Seoul BioHub Global Center has provided significant support, including selection for the 2024 Professional Linkage Program (Scale-up TIPS Investment Track) and participation in the 22nd Interbiz Bio Partnering & Investment Forum.
Through the Professional Linkage Program, we have received expert consulting to prepare for scaling up through the TIPS program and have networked with various stakeholders, fostering valuable business connections.
Participation in the Interbiz Bio Partnering Forum has facilitated investment meetings and discussions, providing crucial support for our ongoing fundraising efforts.
Three reasons why Medi-IoT should receive investment:
Our upcoming products represent the first electronic drugs for eye diseases, combining non-invasive, low-heat light therapy with multi-wavelength and customized light control modules. This innovative approach offers superior usability, portability, and effectiveness, with a significant advantage over existing treatments. We seek investment to support the commercialization and clinical development of these cutting-edge medical devices, aiming to improve eye health management and provide new hope for patients.
We are a specialized startup with expertise in development, H/W, and technology commercialization, supported by a strong advisory group of key doctors, shareholders, and researchers focused on eye disease treatments.
Our market entry strategy includes launching wellness products for eye health management and expanding into electronic therapeutic devices for eye disease treatment. We plan to maximize profits through diversified sales channels and well-prepared market strategies.
MORE FROM THE POST
- [Korean Startup Interview] Mediark: Revolutionizing Patient Diagnosis and Doctor Efficiency with AI-Based Medical Interview System
- [Korean Startup Interview] Beyondmedicine, Pioneering World’s First Digital Therapeutic for TMJ Disorders
- [Korean Startup Interview] Peptirna Therapeutics Aims for Global siRNA Market with Peptide Delivery Platform
- [Korean Startup Interview] MAI Derma’s Cutting-Edge Skincare Tech: The Power of Painless Microneedles
- [Korean Startup Interview] KINGO Bio Challenges Global Giants with Innovative Clean Up Beads Solution ‘GraBeads’
- BioHealth
- Interview
- Korea
- Korean startup
- medi-eye
- Medi-IoT
- mediiot
- SEOUL BIO HUB
- Startup Together
- wow together
- WOWTALK
Share
Most Read
- 1
- 2
- 3
- 4
- 5




Leave a Reply