- Funding Stage : Pre-series A
- Raising amount : KRW 1.5 B
- Desired Fundraising Timeframe : 2Q 2025
Drawing blood to diagnose cancer sounds remarkable. This concept underpins liquid biopsy, a technique that detects cancer using bodily fluids like blood or urine, contrasting with tissue biopsy, which involves extracting cell tissue. While tissue biopsy is ideal for cancer testing, obtaining precise cells is challenging, and post-surgery checks for residual cancer cells would require repeated extractions, causing significant patient distress. In cases like lung cancer, extracting lung cells can cause scarring and severe complications. Despite limitations, liquid biopsy is a steadily growing technology for these reasons.
Liquid biopsy primarily detects circulating tumor DNA (ctDNA) within cell-free DNA (cfDNA) in the blood to identify tumor likelihood. ctDNA consists of fragmented DNA released into the bloodstream as cancer cells die, often containing mutation genes common in cancer patients. For instance, mutations in genes like EGFR, K-RAS, BRCA1/2, or BRAF significantly increase cancer risk, and detecting these in ctDNA is a key goal of liquid biopsy.
Today’s featured company, SimfliBIO, is a liquid biopsy specialist aiming to penetrate markets for companion diagnostics, minimal residual disease (MRD) detection, and early cancer diagnosis with precise gene mutation detection technology. Founded in October 2022 by Professor Sungchul Hohng of Seoul National University’s Physics Department (Biophysics), the company is led by CTO Dr. Chanshin Kang, a KAIST graduate who researched this field with Hohng at SNU.
Diagnosing cancer via ctDNA is complex. The low concentration of ctDNA in blood demands precise detection technology, and if ctDNA is easily detectable, cancer is likely already diagnosed. Post-treatment or post-surgery monitoring of specific mutation genes is critical for determining future treatment paths, requiring highly precise liquid biopsy technology. SimfliBIO developed a novel liquid biopsy technique that detects gene mutations even when ctDNA is just 0.001% of blood content, meaning it can identify one ctDNA among 100,000 cfDNA molecules. This is hundreds of times more sensitive than the prevailing Next Generation Sequencing (NGS) method.
How did SimfliBIO achieve this? The 2024 Nobel Prize in Physiology or Medicine honored researchers who elucidated microRNA’s cellular role, highlighting how Argonaute proteins bind microRNA to regulate protein production via complementary binding with mRNA. Inspired by this, CEO Hohng developed probes that complementarily bind to mutation-causing DNA or RNA sequences, combining them with Argonaute proteins to instantly bind to mutated DNA or RNA, yielding groundbreaking results. These findings were published, patented, and advanced into liquid biopsy technology.

How is SimfliBIO’s technology applied in healthcare? Hohng states, “Our technology will play a pivotal role in companion diagnostics and MRD testing.” Companion diagnostics are crucial for targeted anticancer therapies, as identifying the specific gene mutation causing cancer ensures accurate treatment. For example, treatments vary depending on whether mutations occur in gene A, gene B, or both. SimfliBIO’s 0.001% accuracy in detecting mutation genes aims to deliver essential therapies to patients.
Detecting ctDNA in patients post-treatment or surgery is challenging, yet confirming the presence of minimal residual cancer is critical. This MRD testing demands exceptional sensitivity and accuracy, addressing an unmet need in liquid biopsy where SimfliBIO’s technology excels.
Hohng adds, “Long-term, we aim to make strides in early cancer diagnosis, starting with pancreatic and colorectal cancers.” SimfliBIO has developed technology to simultaneously test four biomarkers—microRNA, ctDNA, proteins, and epigenetic methylation—using just 2ml of blood, positioning it to lead in early diagnosis, Hohng notes.
However, biotech requires rigorous clinical validation. SimfliBIO boldly chose to compare its technology against global biotech leader Guardant Health’s products. In late 2025, it will begin a comparative trial with Guardant360, a non-small cell lung cancer (NSCLC) diagnostic kit, aiming to prove non-inferiority and superior performance in detecting EGFR gene mutations, prevalent in NSCLC patients. Results will be presented at the world’s top three cancer conferences (AACR, ASCO, ESMO) in 2026, marking its global biotech market entry, with plans to expand into pancreatic and colorectal cancer diagnostics.
CEO Hohng, a renowned figure appointed as an SNU professor in 2006 via special recruitment, transitioned from biophysics to develop liquid biopsy technology with molecular precision, challenging the global biotech market. SimfliBIO’s platform technology, applicable to all cancer types, places it among rare Korean biotech firms like Toolgen and LigaChem Bioscience with proprietary technologies.
WOW Partners selected SimfliBIO as a “WOW NEXT 1st Cohort” company, citing its precision and speed surpassing mainstream PCR and NGS, the expertise of Hohng and core team members, and rapid market entry potential via Guardant Health trials.
We look forward to SimfliBIO fostering a world-class Korean company in the global biotech diagnostics market, particularly in critical anticancer diagnostics.

What problem is SimfliBIO trying to solve?
Liquid biopsy diagnoses cancer using bodily fluids, offering a less invasive, faster alternative to tissue biopsy. Applications include early diagnosis and treatment monitoring, with the companion diagnostics market, tied to targeted anticancer drugs, projected to reach USD 41.43 billion (KRW 58 trillion) by 2032.
However, widely used liquid biopsy technologies, PCR and NGS, have limitations:
PCR detects only one gene mutation at a time, unsuitable for analyzing multiple cancer mutations simultaneously.
NGS enables multi-mutation detection but requires over two weeks and is costly, hindering growth.
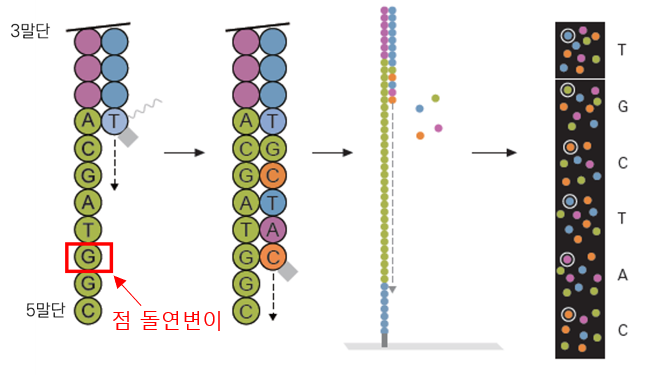
Critically, both struggle to detect mutation DNA below 0.1%. NGS’s difficulty stems from sequencing entire DNA/RNA strands, causing errors, polymerase issues, amplification biases, and signal-to-noise confusion, where low-concentration mutation signals are masked.
How does SimfliBIO solve this?
NGS scans entire DNA/RNA sequences, leading to lengthy analysis and errors. We scan only mutation regions, triggering a fluorescent signal, like an alarm, for instant results. Periodic fluorescence blinking enhances detection accuracy, overcoming traditional limitations.

Technically, the RNA-induced silencing complex (RISC), combining Argonaute proteins with microRNA, rapidly binds to complementary mRNA. We created probes with complementary sequences to mutation regions, binding them with Argonaute proteins to form complexes that swiftly attach to target DNA, emitting fluorescence for precise mutation detection. This enables rapid, low-concentration mutation detection, surpassing PCR and NGS limits.
What are our competitive advantages and technical strengths compared to competitors?
In companion diagnostics, liquid biopsy detects multiple somatic cancer mutations via sequencing to select therapies. NGS dominates due to its multi-mutation capability, unlike PCR. However, NGS requires over two weeks and cannot detect mutations below 0.1%, a critical barrier to market growth.
Our technology delivers results in six hours and detects mutations below 0.001%, offering unmatched speed and accuracy. This enables companion diagnostics for stage 2 cancers, MRD, and early diagnosis, unachievable with PCR or NGS.
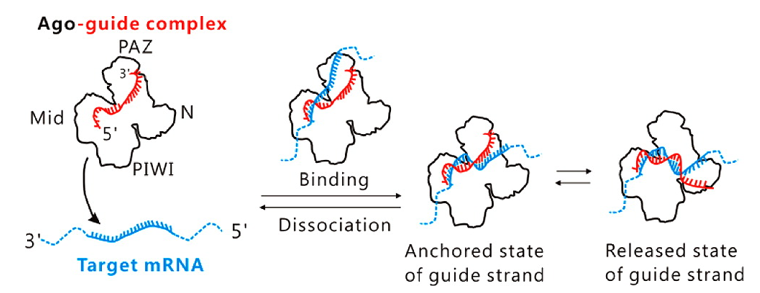
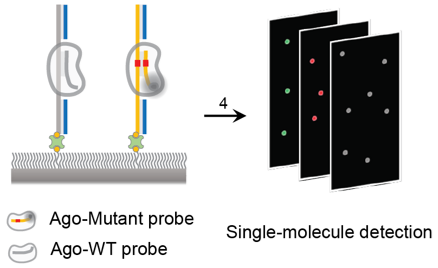
What products/services do we offer, and what is their current status?
Our primary pipeline is NSCLC companion diagnostics. The WHO estimates 515,010 NSCLC treatment patients in G7 countries in 2020, rising to 591,780 by 2030. At USD 2,857 (KRW 4 million) per NGS test, the market is approximately USD 1.43 billion (KRW 2 trillion). Our NSCLC companion diagnostics will operate as a Laboratory Developed Test (LDT) service, a common approach.
We’ve completed 50 probes for EGFR mutations, observed in 30–40% of NSCLC patients, targeted by anticancer drugs. This year, we’ll conduct comparative trials against Guardant360, which holds 80% of the NSCLC diagnostic market, with Seoul National University Hospital, Samsung Medical Center, and the National Cancer Center. Comparative trials are essential to validate new technologies for LDT services, ensuring analytical sensitivity, specificity, accuracy, and reproducibility.
We’ll present results at 2026 global cancer conferences to secure U.S. and European partners for commercialization. In the U.S., LDT requires a CLIA-certified lab, costing ~USD 1.43 million (KRW 2 billion) for acquisition. Domestically, we’ll establish an LDT lab and seek KFDA approval. Our trials focus on proving superiority over existing products, enhancing commercialization likelihood.
What is our target market size, and who are our core customers?
The NSCLC companion diagnostics market is ~USD 1.43 billion (KRW 2 trillion), with Guardant360’s annual revenue at ~USD 0.57 billion (KRW 0.8 trillion). We plan to commercialize diagnostics for colorectal cancer (CRC), pancreatic cancer, and breast cancer, plus screening for pancreatic and NSCLC. Developing detection probes enables diverse diagnostics and MRD applications. For early diagnosis, we’ll target CRC, proving superiority over fecal occult blood tests for insurance and national screening inclusion.
What is our business model?
Our companion diagnostics will launch via LDT services, avoiding hardware or kit sales. We’ll build in-house labs with total internal reflection fluorescence microscopes and produce kits internally, bypassing commercialization hurdles. As the market grows, we’ll commercialize hardware and kits for external sales.
What are SimfliBIO’s achievements?
As of May 2025, we’ve developed a total internal reflection fluorescence microscope and diagnostic kit analysis program with 100% proprietary technology. The equipment is manual but requires automation for multi-detection, which we’ll address post-investment with specialized hires. We can detect mutation DNA at 0.001% concentration, with secured patents and published validation. EGFR mutation probes were completed in May 2025, with optimization expected in 2–3 months, enabling Guardant360 comparative trials.
What is the SimfliBIO team’s competitiveness?
CEO Sungchul Hohng and CTO Chanshin Kang lead our technology development, introducing novel liquid biopsy solutions. Hohng, an SNU professor since 2006, is a global leader in single-molecule biophysics. Kang, with a KAIST degree, has researched this technology with Hohng. Dr. Kyungwon Kim, joining as COO in August, a molecular immunology Ph.D., brings 13 years of diagnostic panel and regulatory expertise. Dr. Changwon Park, our CSO, with a KAIST molecular genetics Ph.D., will leverage experience from Illumina Korea and Avellino Lab to secure strategic partners.
Why should we receive investment? Three reasons!
Our paradigm-shifting technology ensures low failure risk and market leadership potential, unlike typical drug startups.
Successful 2025 comparative trials could enable immediate licensing to multinationals, offering investor exits, as global firms seek high-sensitivity diagnostics.
Our trials require less capital than drug trials, with predictable outcomes via pre-clinical probe optimization, reducing commercialization risks.
MORE FROM THE POST
- [Korean Startup Interview] CrossPoint Tackles Key ADC Market Issue with Fc Silencing Tech, Attracting Global Biotech Interest
- [Korean Startup Interview] Beyondmedicine, Pioneering World’s First Digital Therapeutic for TMJ Disorders
- [Korean Startup Interview] Peptirna Therapeutics Aims for Global siRNA Market with Peptide Delivery Platform
- [Korean Startup Interview] KINGO Bio Challenges Global Giants with Innovative Clean Up Beads Solution ‘GraBeads’
- [Korean Startup Interview] OCULIGHT: Shining a Light on Safer, More Accurate Cataract Surgeries
Share
Most Read
- 1
- 2
- 3
- 4
- 5




Leave a Reply