- Funding Stage : Seed
- Raising amount : KRW 200 M
- Desired Fundraising Timeframe : 2Q 2025
Proteins, formed by chains of amino acids folding into 3D structures, perform diverse functions in cells and have become a key target for modern drug development. When genetic mutations lead to “disease-causing proteins,” conditions like cancer or other diseases arise, prompting the development of technologies to neutralize these proteins.
What if, instead of treating these proteins after they’re formed, we could prevent their creation entirely? This idea gave rise to siRNA, or small interfering RNA.
Genes in the cell nucleus produce messenger RNA (mRNA) containing instructions for specific proteins, which is sent out for ribosomes to translate into proteins. If an mRNA carries instructions for a disease-causing protein, introducing siRNA into the cell can bind to and block it, preventing protein production. The discovery of siRNA’s mechanism earned two scientists a Nobel Prize in 2006, and its proven efficacy has driven numerous companies to develop siRNA therapies.
The challenge lies in delivering siRNA into cells effectively. Today’s featured company, Peptirna Therapeutics, is developing a “peptide delivery platform” to address this issue.
siRNA struggles to enter cells because it carries a negative charge (-). Nucleotides in DNA and RNA are negatively charged, as are cell membranes, making penetration difficult. Peptirna overcomes this by creating a peptide that binds to siRNA. This positively charged (+) peptide forms a neutral complex, enabling cell entry.
Alternatively, lipid nanoparticles (LNPs) can create such complexes, but their patents are held by global biotech firms, requiring hefty licensing fees. For instance, Moderna faced a major patent lawsuit from Arbutus Biopharma in 2021 over LNP use in its COVID-19 mRNA vaccine and lost. Additionally, LNPs are unstable, necessitating ultra-low-temperature cold chains for transport and storage.

Due to these issues, most global siRNA drug developers use GalNAc, a molecule that binds strongly to specific liver cell receptors, making it difficult to deliver therapies to non-liver cells. As a result, most siRNA drugs target liver diseases or related conditions.
Another hurdle is that siRNA, once inside a cell, struggles to escape the endosome surrounding it. Peptirna’s peptide is designed to facilitate endosomal escape, enabling effective action within the cell. CEO Byung-il Kim emphasizes, “Our platform, Pepticellplex, is our greatest strength, comprehensively addressing LNP complexity, delivery to non-liver tissues, and endosomal escape.”
CEO Byung-il Kim, who earned a master’s in molecular biology from Korea University, has dedicated over 13 years to peptide material research. Driven by entrepreneurial ambition, he founded Peptirna to develop an siRNA drug delivery platform based on his peptide technology.
The global leader in siRNA therapeutics, Alnylam in the U.S., focuses primarily on liver diseases. If Peptirna’s delivery technology is adopted, it could serve Alnylam and other siRNA drug developers as customers.
However, this may seem ambitious for a small Korean biotech startup. Thus, CEO Kim is pursuing a practical short-term business model alongside long-term goals, developing delivery systems for cosmetic ingredients. He explains, “Popular cosmetic ingredients like PDRN, NAD, hyaluronic acid, collagen, and ATP are negatively charged, allowing binding to Pepticellplex. At 20 nanometers, they’re small enough to penetrate skin.” Positive experimental results have attracted manufacturing requests from leading Korean cosmetic firms. Success in this model would be significant and provide a strong foundation for Peptirna’s mid-to-long-term growth.
Peptirna’s modest office in Suwon is well-equipped for experiments, with ongoing research creating a dynamic environment. Each meeting with CEO Kim reveals new achievements, leaving a strong impression.
For these reasons, WOW Partners selected Peptirna as a member of “WOW NEXT 1st Cohort”, considering the scalability of the siRNA therapeutics market, the growth potential of its delivery platform, and the practicality and diligence in developing cosmetic ingredient delivery systems.
A small company doesn’t mean small dreams. As we look at Peptirna, we anticipate a Korean biotech making its mark in the growing global siRNA market.

What problem is Peptirna trying to solve?
We aim to solve the biggest hurdle for nearly all RNA therapeutics: the difficulty of delivering drugs into cells.
Among them, siRNA (small interfering RNA) is an excellent molecule that strongly inhibits messenger RNA (mRNA) responsible for disease-causing proteins, blocking their production at the source. However, its double-stranded structure results in a large molecular weight, and its strong negative charge, like the cell membrane, prevents it from penetrating cells.
Despite siRNA’s clear mechanism, developing it into a therapy requires effective drug delivery technology.
How does it solve this problem?
The primary reasons siRNA cannot penetrate cell membranes are its molecular weight and negative charge.
To address this, we combine siRNA with a positively charged material to form nano-sized particles. Using our cell-penetrating peptide design technology, we screened peptides with components for electrostatic binding to siRNA (like magnets) and nanoparticle formation, securing a highly efficient drug delivery platform.
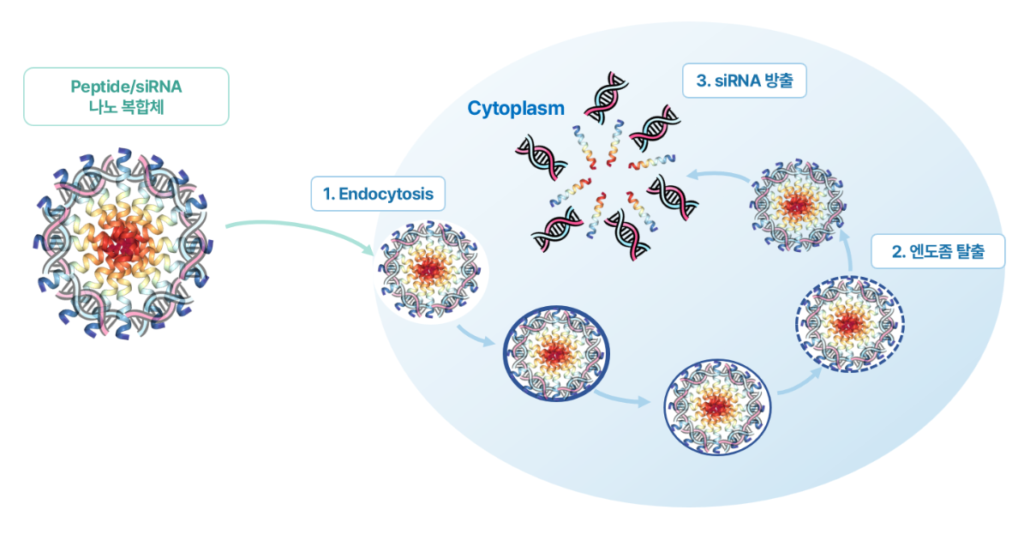
What are our competitive advantages and technical strengths compared to competitors?
Currently, FDA-approved siRNA drug delivery systems are mainly lipid nanoparticles (LNPs) and GalNAc-siRNA conjugates.
LNP materials are patented by U.S. firms like Alnylam and global pharma, making it nearly impossible to develop without infringement. Even if developed, LNPs require ultra-low-temperature cold chains and primarily deliver to the liver, limiting their scope.
GalNAc, an amino sugar derivative, binds 50 times more strongly to ASGPR receptors on liver cells than galactose, allowing subcutaneous delivery to the liver. Most companies develop GalNAc-siRNA conjugates for liver disease treatments.
Our proprietary peptides avoid patent infringement and toxicity issues. As nanoparticles, they enable passive targeting to non-liver tissues. Additionally, attaching specific ligands like antibodies to our product’s surface allows active targeting to desired cells or tissues, distinguishing our platform.
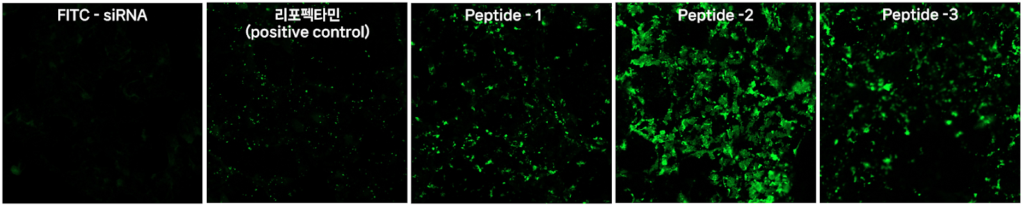
What products/services do we offer, and what is their current status?
Our peptide-based drug delivery platform has three key features.
First, it forms nanoparticles under 100 nanometers, enabling rapid cell entry and siRNA delivery to the cytoplasm.
Second, our positively charged peptides form electrostatic bonds with negatively charged siRNA, allowing multiple target siRNAs to be loaded simultaneously, maximizing therapeutic efficacy. Alnylam’s Gemini platform and Sirnaomics’ nanoparticle with dual siRNAs are similar examples in global trials.
Third, by introducing specific amino acid functional groups to our peptide’s end, we can attach desired ligands to the nanoparticle surface, enabling drug delivery to specific cells or tissues, addressing the liver-centric focus of current siRNA therapies.
What is the target market size, and who are the core customers?
Traditional small-molecule and antibody drugs target disease-causing proteins after they’re formed. siRNA therapies, by degrading mRNA before protein expression, can address “undruggable” targets unattainable by conventional drugs.
With abundant global research on disease-related genes and proteins, siRNA therapies for previously untreatable targets are highly attractive to big pharma firms. siRNA drugs often see technology transfers exceeding USD 714.29 million (KRW 1 trillion) in preclinical or Phase 1 stages. There’s also strong interest in platforms delivering siRNA to non-liver tissues. For example, DTx Pharma, founded in 2017, developed a CNS-optimized siRNA platform and was acquired by Novartis in 2023 for USD 928.57 million (KRW 1.3 trillion).
We aim to secure robust intellectual property for our siRNA-optimized peptides, targeting technology transfers or acquisitions by interested pharma and biotech firms from the preclinical stage.
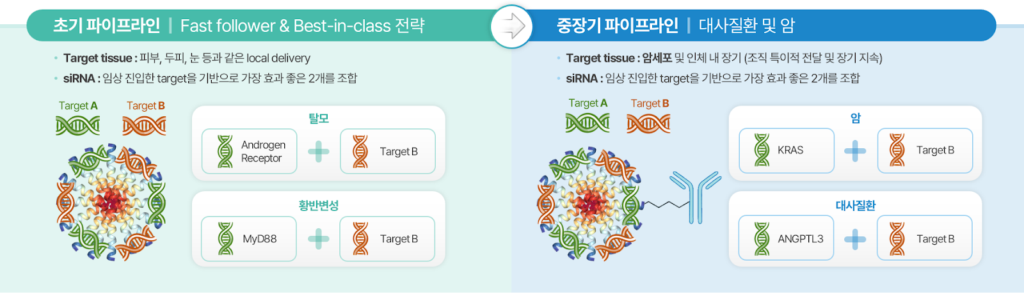
What is our business model?
Our drug delivery platform is geared toward siRNA therapeutics long-term, but we also need short-term cash flow strategies.
As our peptide complexes are nano-sized, they’re used for skin delivery in cosmetics. The cosmetics industry prioritizes delivery systems for proven ingredients over new ones. Our peptide nanoparticles, carrying active substances like nucleotides similar to siRNA, showed strong efficacy in skin trials, leading to B2B sales for products with various low- to high-molecular-weight ingredients.
Starting with skin delivery efficacy, we’ll accelerate siRNA therapy development for localized areas like skin and eyes, later expanding to high-impact fields like cancer and autoimmune diseases.
What are our team’s achievements?
In 2023, Sungkyunkwan University’s Startup-Centric University selected our siRNA delivery project, leading to our incorporation. In 2024, we earned recognition for our technology in Gyeonggi Center for Creative Economy’s Open Innovation Program and the Ministry of SMEs and Startups’ Technology Development Program, advancing our R&D.
As of 2025, our skin delivery technology has attracted significant interest from Korea’s largest cosmetic manufacturers and global brands, with sales efforts underway for pharmaceutical dermocosmetic brands. We’ve developed raw materials with eight active ingredients, expecting significant revenue from the second half of this year.

What is the Peptirna team’s competitiveness?
Our extensive experience in developing peptides and derivatives allows us to design peptides in novel ways, securing a platform to deliver siRNA into cells without toxicity or side effects for promising biotech drugs.
Our ability to attach ligands for targeted delivery to specific cells or tissues sets us apart from existing delivery systems.
Finally, peptides are relatively safe and widely used in cosmetics. Our platform’s dual use for siRNA delivery and cosmetic ingredient delivery enables rapid revenue generation, a key advantage for an early-stage biotech startup.
Why should we receive investment? Three reasons:
First, as a delivery platform for siRNA—a Nobel Prize-validated, highly effective molecule—we have a lower failure risk and higher success potential than drug development firms.
Second, our platform addresses the biggest unmet need in siRNA therapy: delivery to non-liver tissues, expanding beyond the liver-focused market.
Third, our magnetic-like binding of peptides and siRNA allows multiple siRNAs to be delivered simultaneously without chemical modification, drastically reducing development time and costs.

MORE FROM THE POST
- [Korean Startup Interview] Beyondmedicine, Pioneering World’s First Digital Therapeutic for TMJ Disorders
- [Korean Startup Interview] KINGO Bio Challenges Global Giants with Innovative Clean Up Beads Solution ‘GraBeads’
- [Korean Startup Interview] OCULIGHT: Shining a Light on Safer, More Accurate Cataract Surgeries
- [Korean Startup Interview] MEDI-EYE: Revolutionizing Eye Health with Advanced Light Therapy
- [Korean Startup Interview] Mediark: Revolutionizing Patient Diagnosis and Doctor Efficiency with AI-Based Medical Interview System




Leave a Reply